Подробнее об особенностях работы умной аналитики SellerStats для каждого из маркетплейсов — на сайте
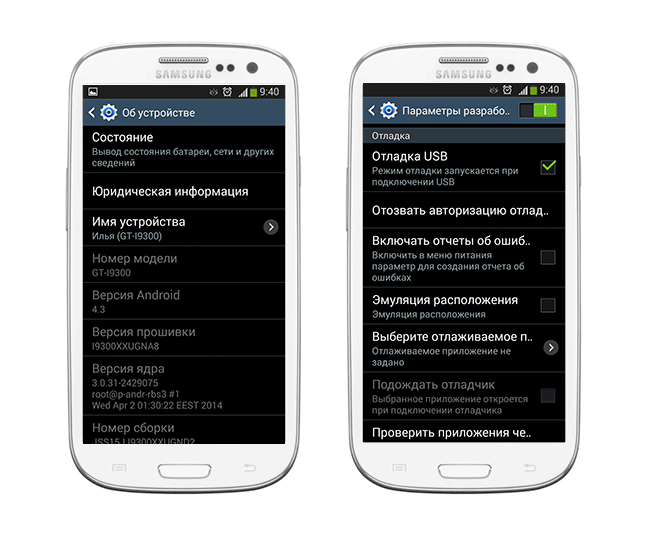
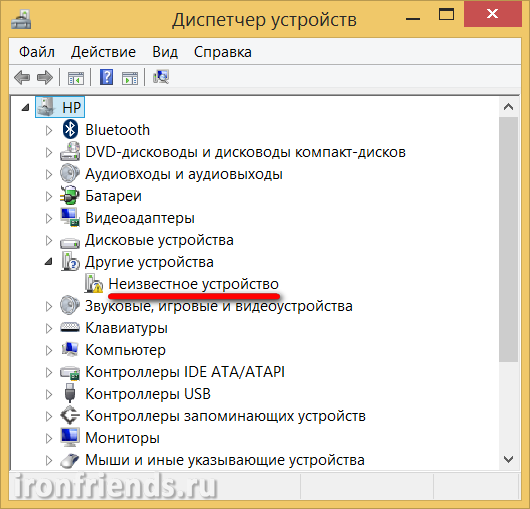
Что делать, если компьютер не видит телефон с Андроид через USB, основные способы устранения
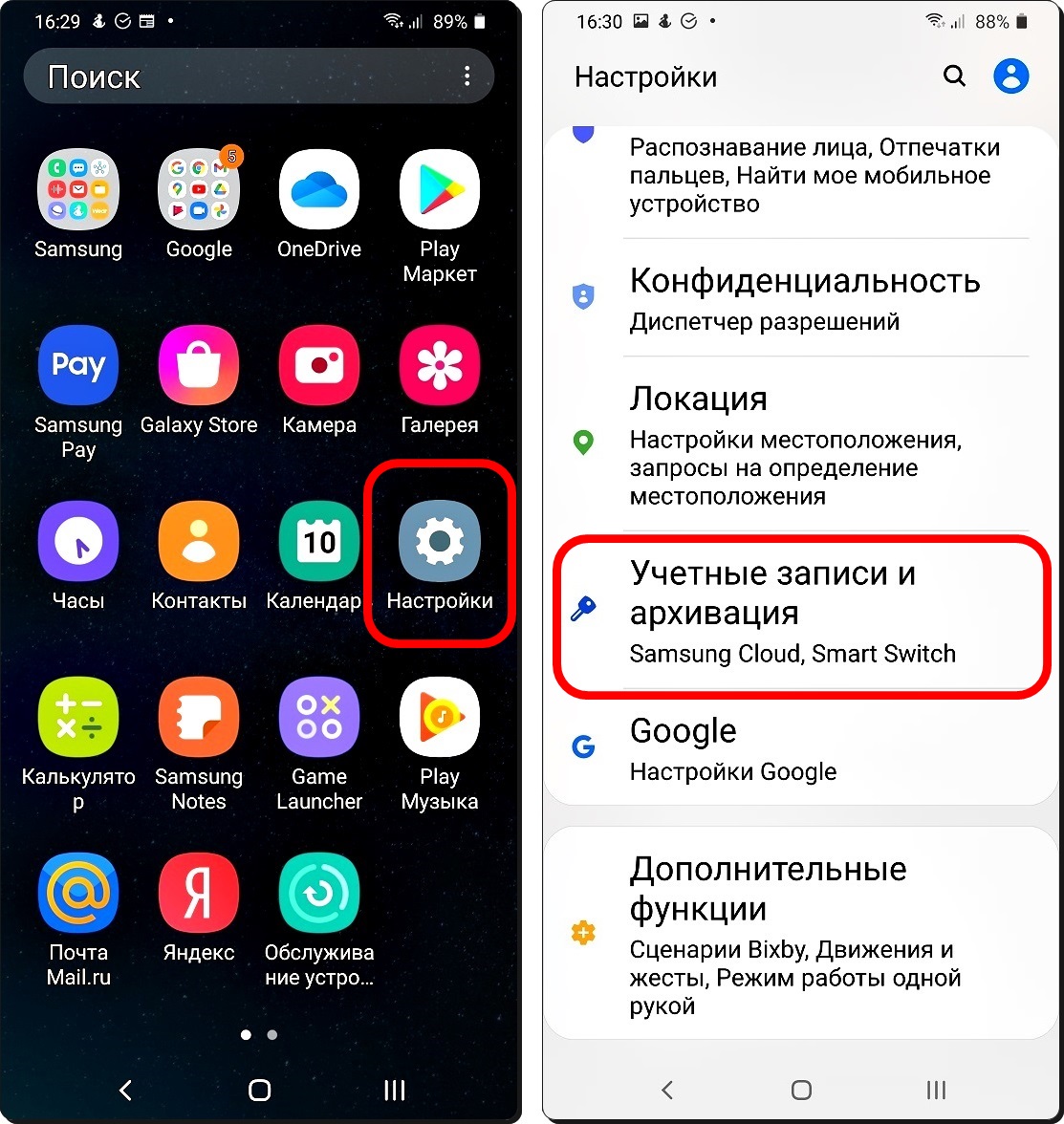
Что лучше выбрать для себя — Google Pay или Samsung Pay. Подробное сравнение приложений
Прошивать Samsung очень легко, но не зная определенных правил можно получить отличный смартфон-орехокол! Чтобы
Баланс аппаратных компонентов и уровня производительности, заложенный в конструкции отдельных устройств Android, иногда вызывает
Вашему вниманию пошаговая инструкция по прошивке телефона Самсунг Galaxy S4 на Android 4.4.2. Производительная
Смартфон Samsung A40 может перестать заряжаться в силу самых разных причин. Чаще всего это
Как восстановить удалённые контакты на телефонах Samsung Galaxy Иногда из-за различных причин и обстоятельств
В процессе использования ноутбуков от разных производителей пользователи часто сталкиваются с различными проблемами и
Сделать обреку видео на устройстве Android можно средствами самой системы, с помощью бесплатных онлайн
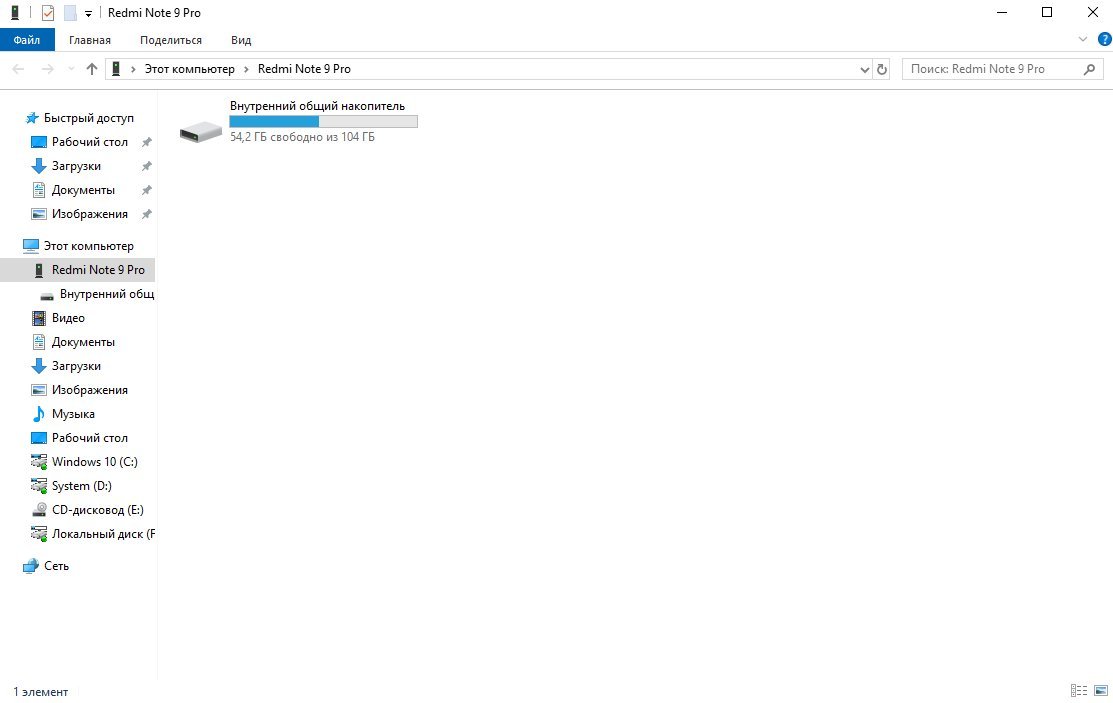
Чтобы подключить флешку к телефону, необходимо убедиться что накопитель отформатирован под FAT32. Рекомендуем проверить
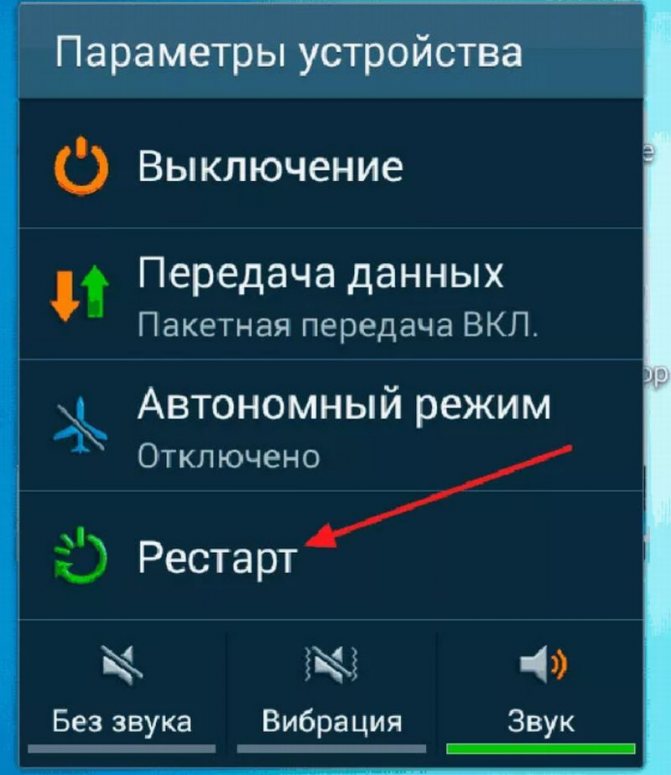
Читайте инструкции, как можно перезагрузить Андроид смартфон. С помощью кнопок, основных настроек и сторонних
Разгоняем Samsung Galaxy Y S5360 (оверклокинг!) - Android, overclocking, Samsung Galaxy Y, samsung spica,
Сегодня я пошагово объясню, как обновить Samsung Galaxy S3 до Android 7.1 Nougat. Учебное
➥Как проверить на оригинальность Самсунг? ➥В статье Вы узнаете, какие смартфоны линейки Samsung подделывают
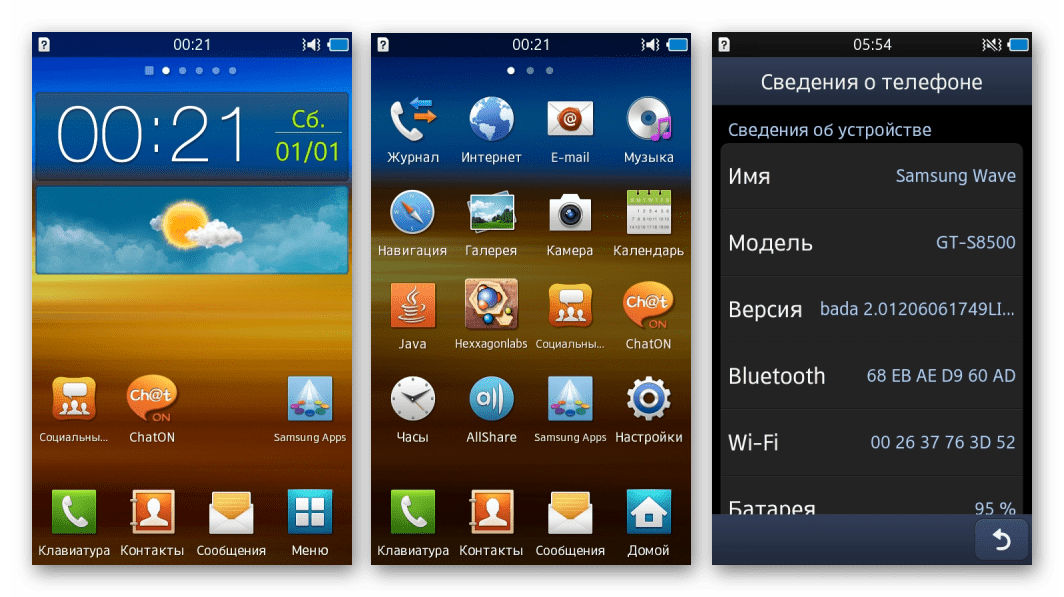
Прошивка смартфона Samsung Wave GT-S8500 дозволяє досить просто встановити дві ОС - Bada і
Несмотря на почтенный возраст Samsung Galaxy Star Plus GT-S7262, прошивка смартфона позволяет восстановить работоспособность
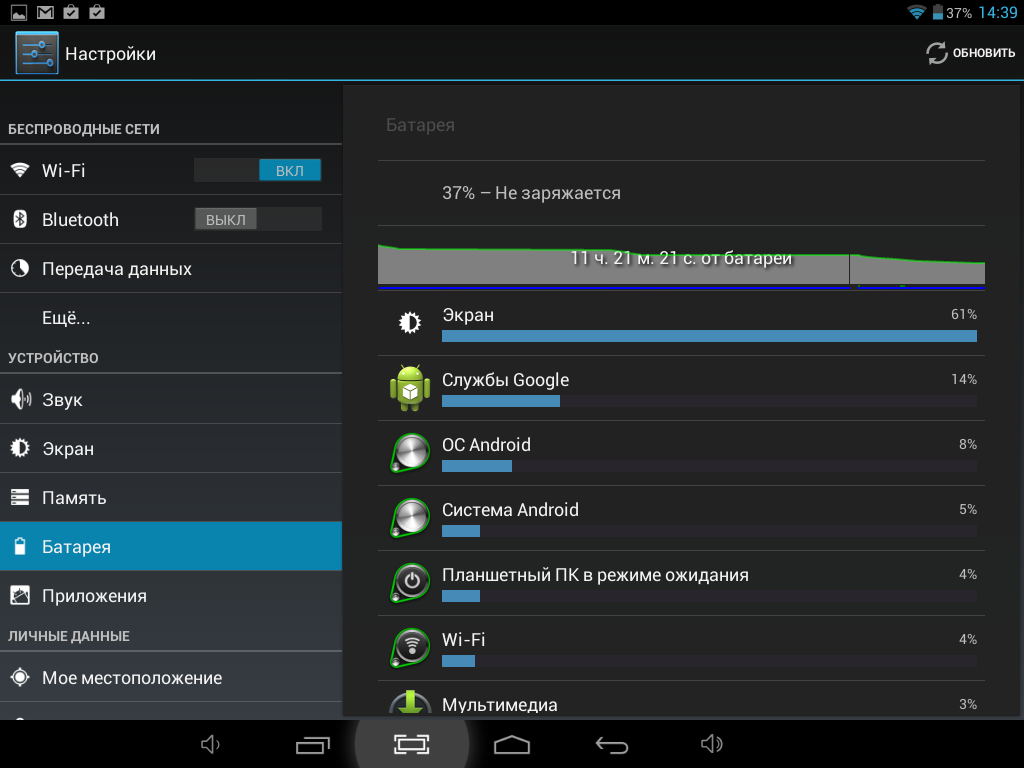
Обновить Андроид на планшете можно по воздуху, автоматически, через SD-карту, скачав с сайт производителя,
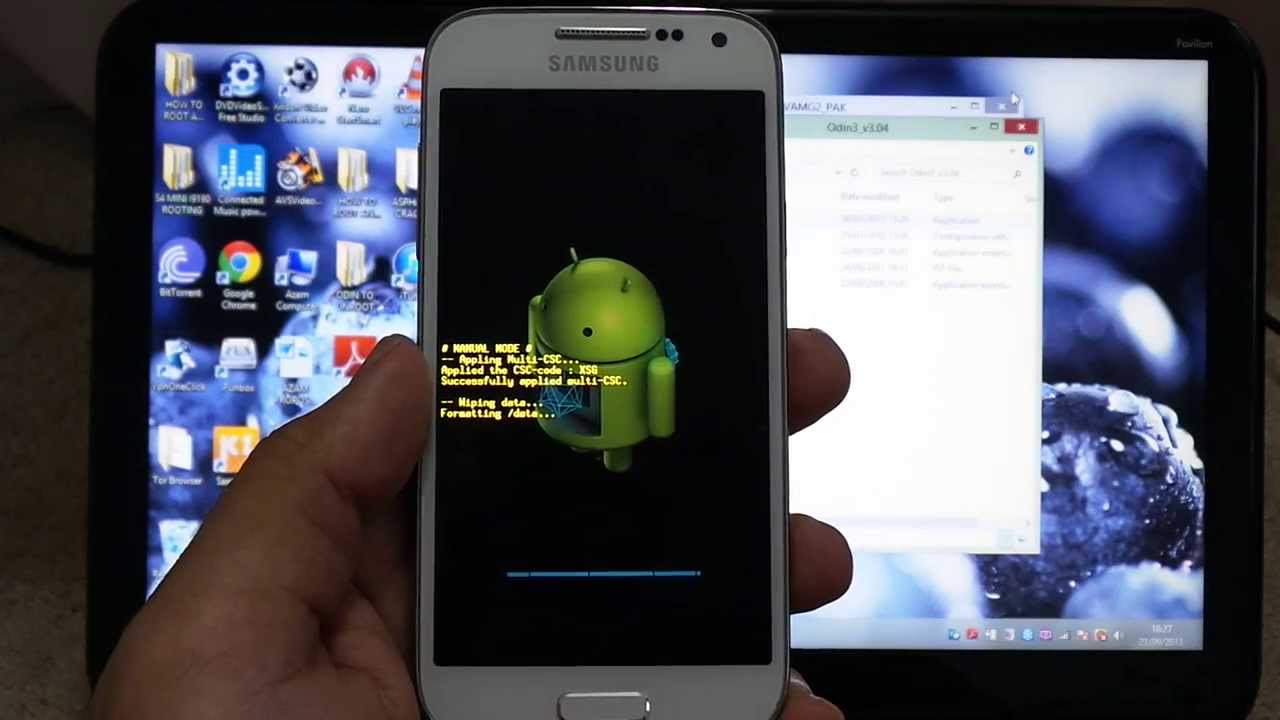
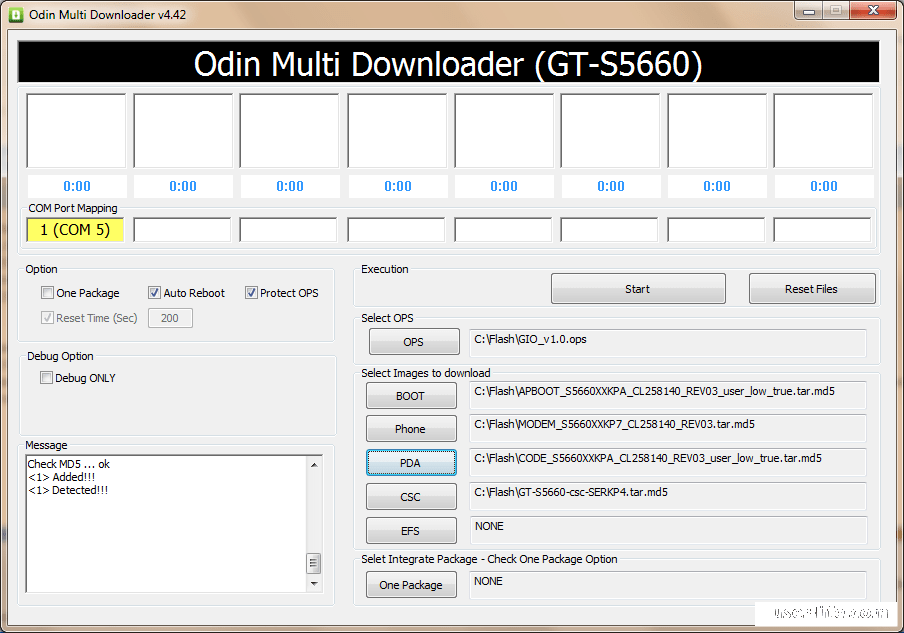
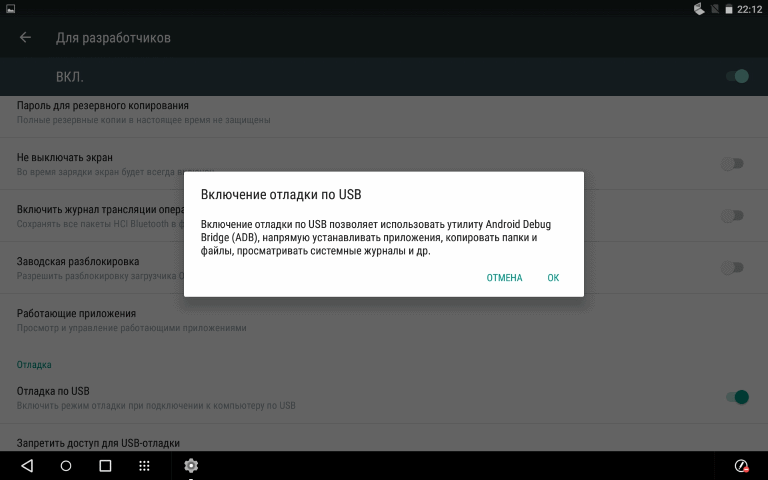
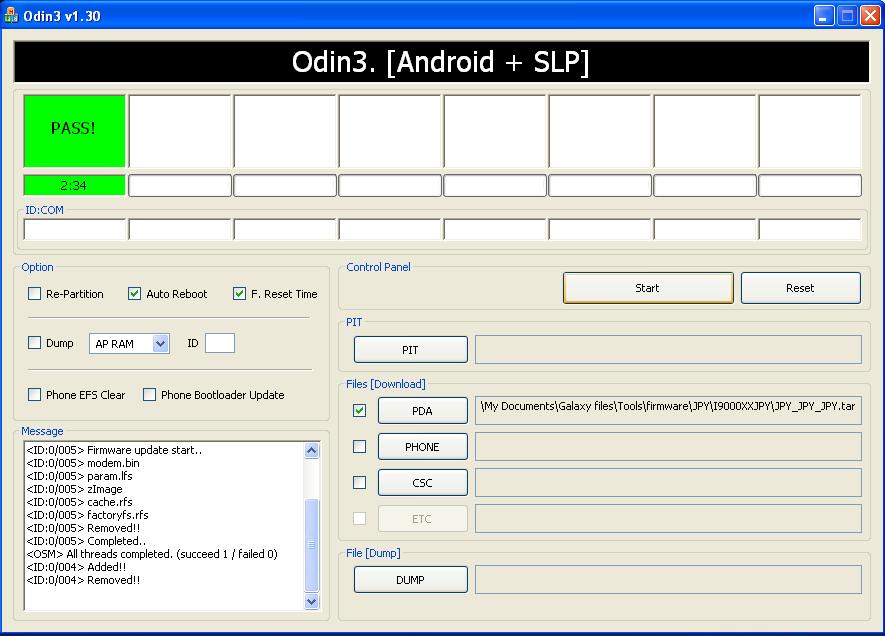
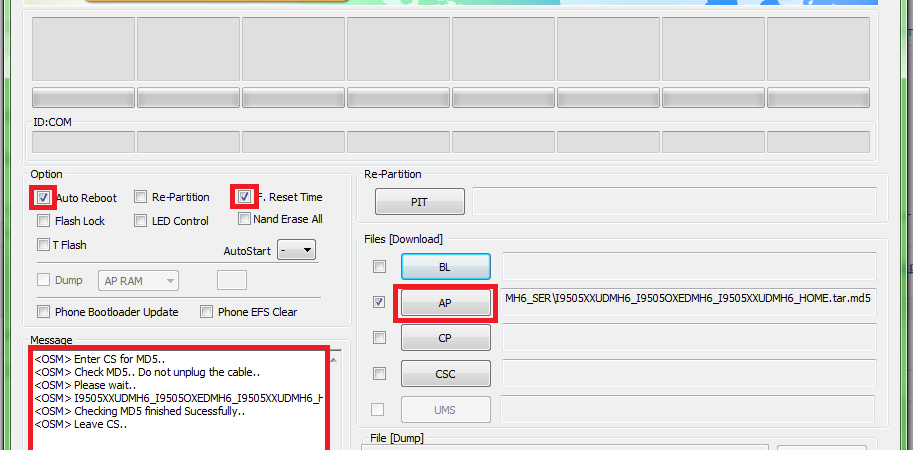
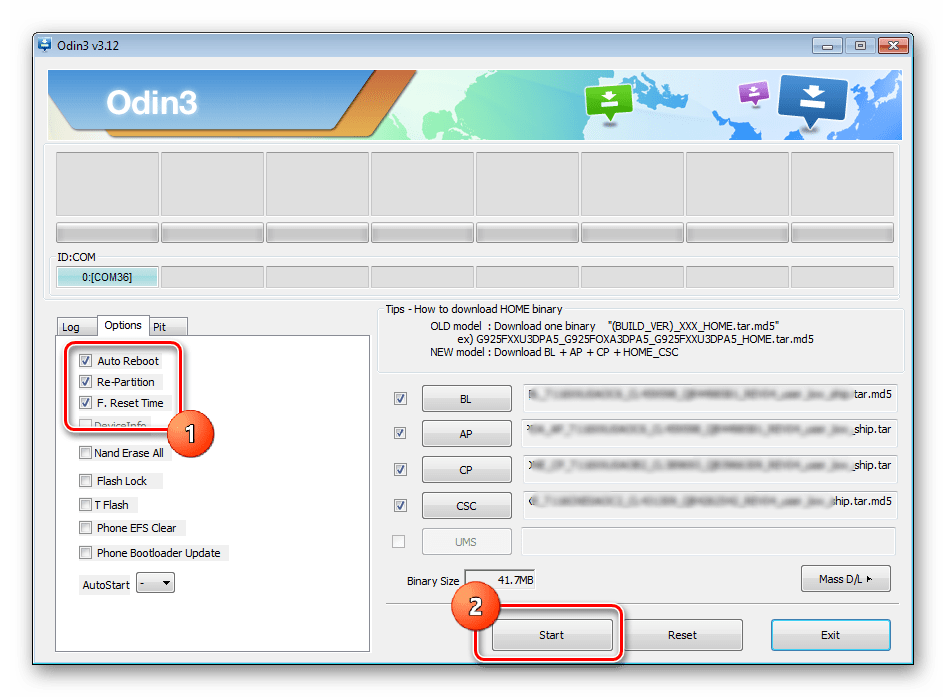
Подробная инструкция по прошивке любого смартфона Samsung через программу ODIN с помощью компьютера. Где
Установка драйверов для ноутбука Samsung N150 Plus Установка драйверов для ноутбука Samsung N150 Plus
Нормальное функционирование ноутбука Samsung NP355V5C без драйверов невозможно. Вы можете без проблем скачать и
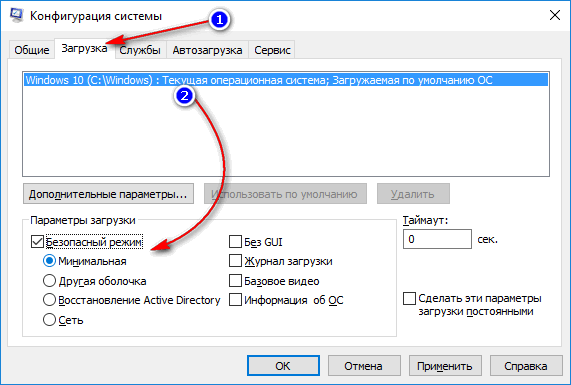
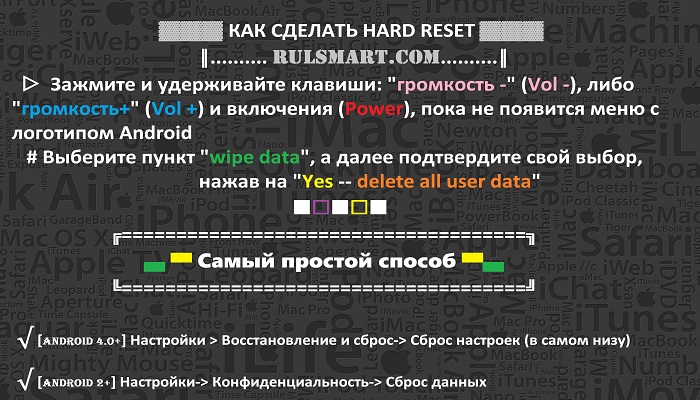
Что делать , если слетел загрузчик андроид? Как восстановить Bootloader? Основные причины поломки и
В момент установки прошивки на Samsung все время выбивает ошибки? Как это все исправить
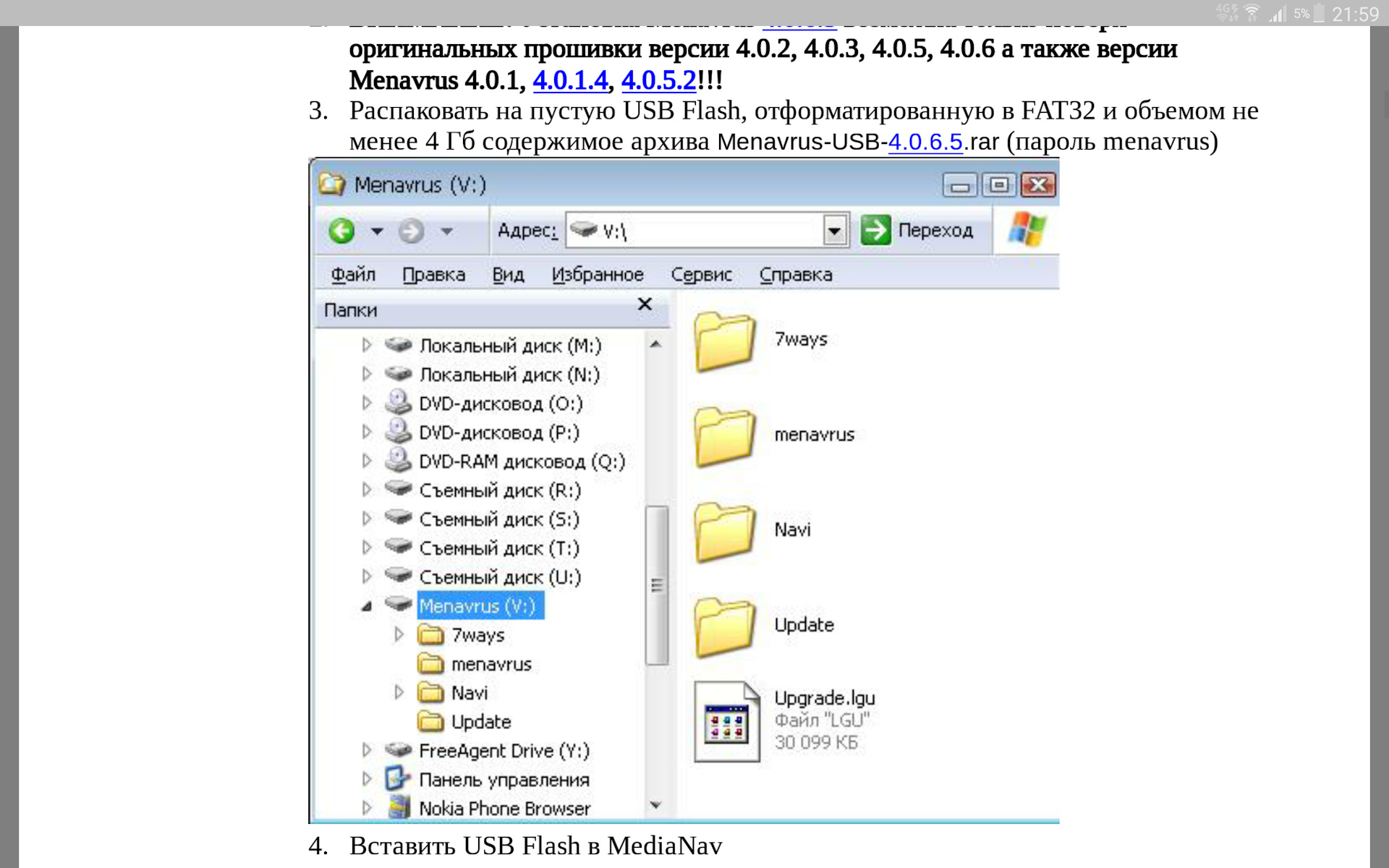
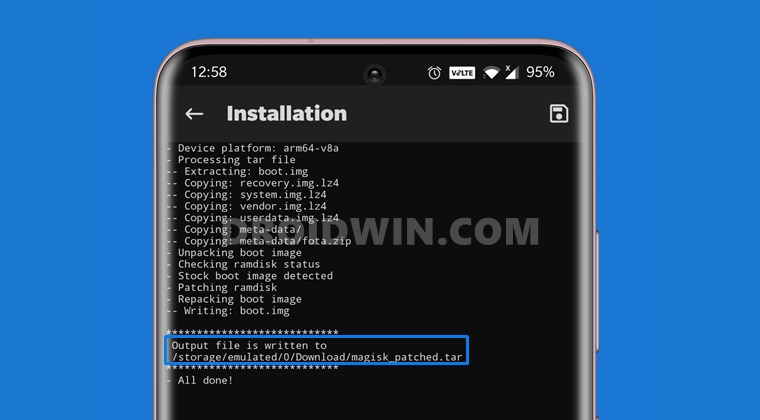
Вам интересно экспериментировать с установками различных версий прошивок (не только официальных от производителя, но
Обновление, прошивка или перепрошивка смартфонов Samsung серии Galaxy позволит вам изменить версию операционной системы
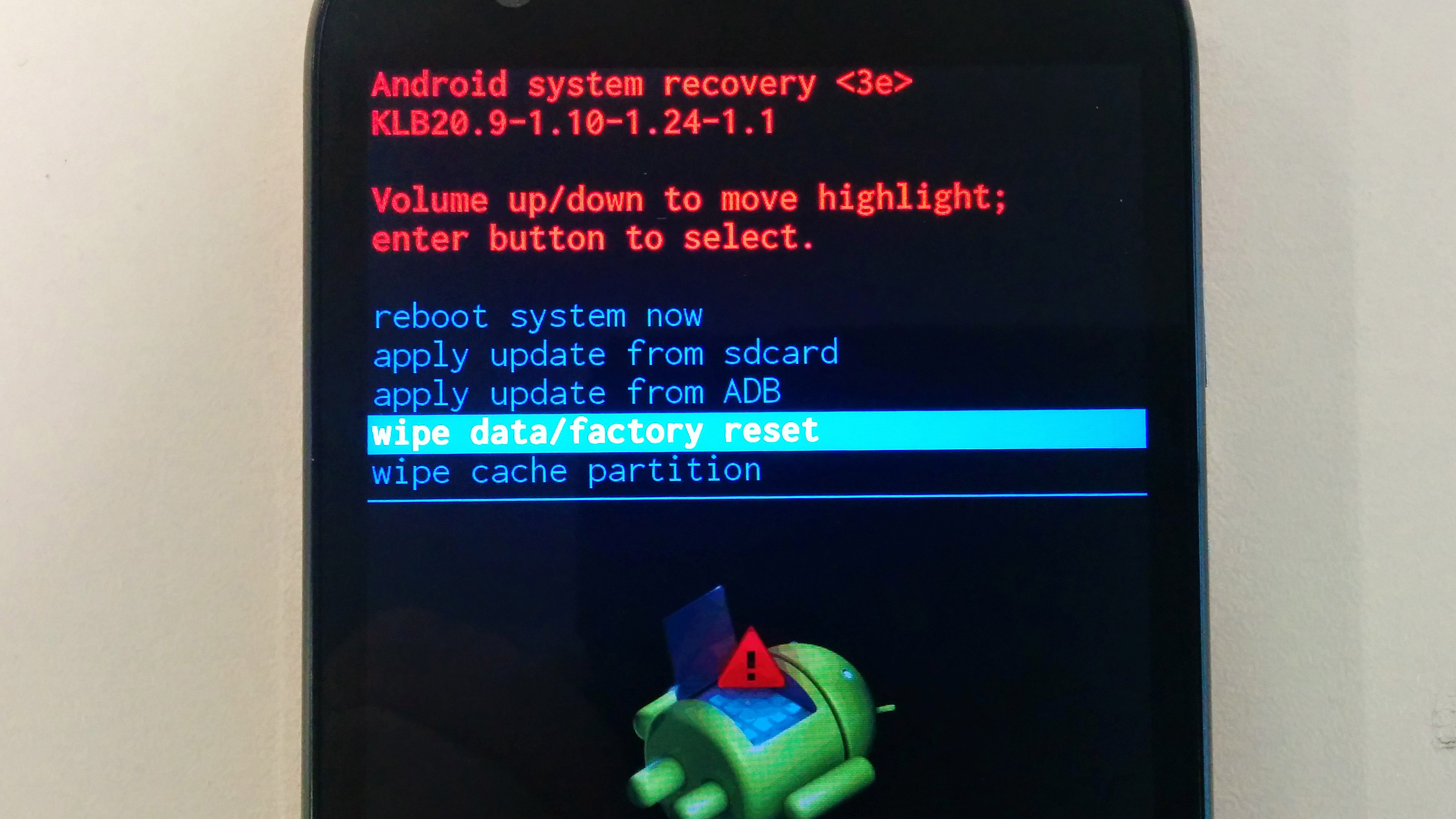
Не прошивается телефон через odin пишет fail | Портал о телефонах и сотовых операторах.
Компания Samsung закрыла производство телефонов в Китае. А в каких странах компания осуществляет сборку
In this detailed tutorial, we will show you detailed steps on how to download